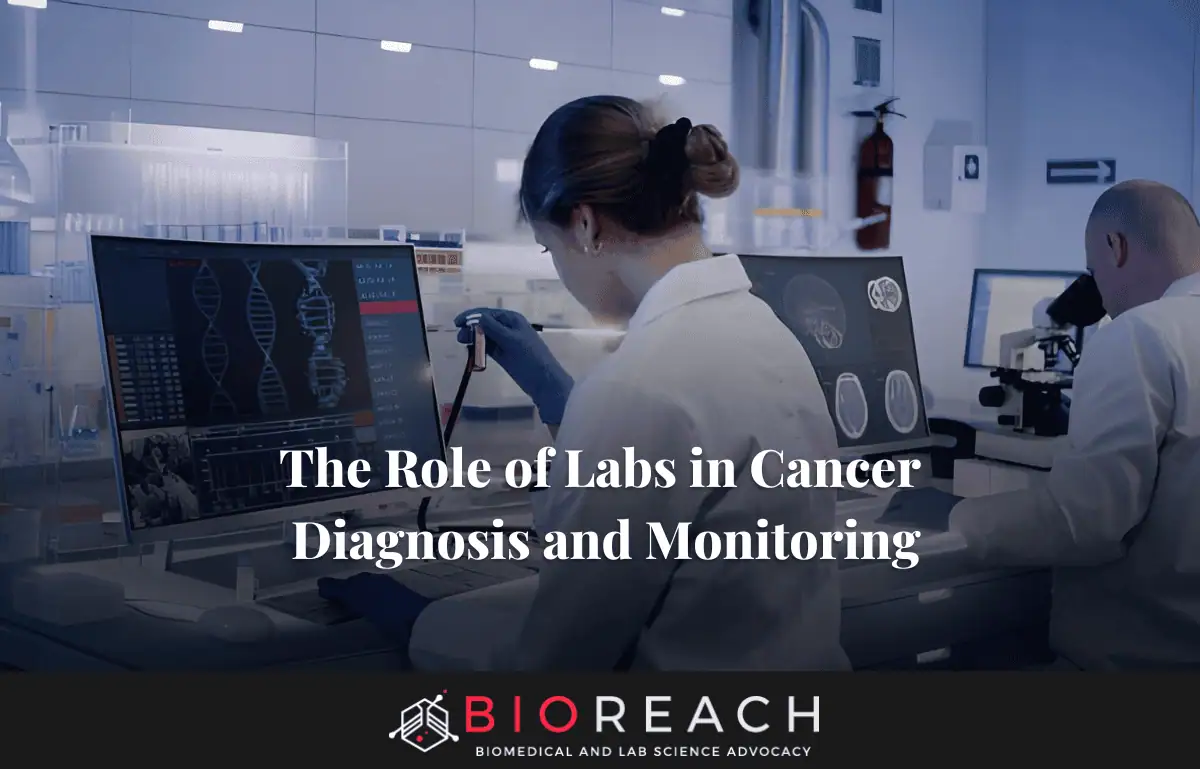
In the shadowed halls of high-volume pathology facilities, labs in cancer diagnosis and monitoring play a silent yet vital role in humanity’s ongoing battle against the disease. Here, fluorescent markers illuminate tumor cells under a microscope, and robotic arms pipette precise volumes of blood serum, each motion a step toward clarity and hope. Every slide and vial represents a patient’s chance for early detection or sustained remission.
As of 2025, cancer remains a formidable adversary: in the United States alone, an estimated 2,041,910 new cases will be diagnosed this year, with 618,120 lives lost to the disease. Globally, the burden is even heavier, with projections nearing 22 million new diagnoses annually, driven by aging populations and lifestyle factors. Yet amid this grim forecast, clinical laboratories stand as unsung heroes, delivering the molecular intelligence that transforms vague symptoms into targeted therapies.
The role of labs in cancer care extends far beyond routine blood work. They are the crucibles where raw biological samples blood, tissue, urine, and even exhaled breath, are transmuted into actionable data. From identifying oncogenic mutations via next-generation sequencing (NGS) to tracking minimal residual disease (MRD) through circulating tumor DNA (ctDNA) analysis, labs bridge the gap between suspicion and certainty. This precision has tangible impacts: early lab-driven diagnoses can boost five-year survival rates from under 20% for metastatic cancers to over 90% for localized ones. But as technologies evolve, so do the complexities, ethical dilemmas in genetic privacy, disparities in access, and the relentless push for sensitivity in an era of overtreatment.
This article dissects the multifaceted contributions of laboratories to cancer diagnosis and monitoring. We explore foundational diagnostic tests, cutting-edge biomarkers, real-time treatment surveillance, transformative technologies, compelling case studies, persistent challenges, and visionary trends shaping oncology’s future. By illuminating these elements, we reveal how labs are not merely supportive but central to redefining cancer as a manageable chronic condition rather than an inevitable death sentence.
The Foundational Role of Labs in Cancer Diagnosis and Monitoring
Cancer diagnosis begins with a whisper, a persistent cough, an unexplained lump, or fatigue that defies rest. But confirmation demands the rigor of the lab, where tests dissect the body’s betrayal at cellular and molecular levels. Traditionally, diagnosis relied on histopathology: tissue biopsies stained with hematoxylin and eosin (H&E) to reveal malignant architecture. In 2025, however, labs integrate multimodal approaches, combining cytology, immunohistochemistry (IHC), and flow cytometry to achieve diagnostic accuracy exceeding 95% for common solid tumors.
At the core are screening and diagnostic assays. For breast cancer, the most frequently diagnosed malignancy in women (projected 313,510 U.S. cases in 2025), labs process mammogram-guided core biopsies, employing estrogen receptor (ER), progesterone receptor (PR), and HER2 IHC panels to classify subtypes. These markers dictate therapy: triple-negative breast cancers (ER/PR/HER2-negative) demand aggressive chemotherapy, while HER2-positive cases respond to trastuzumab. Similarly, in colorectal cancer (anticipated 153,620 U.S. cases), fecal immunochemical tests (FIT) detect occult blood, prompting colonoscopies and subsequent lab confirmation via KRAS/BRAF mutation profiling to guide anti-EGFR therapies like cetuximab.
Liquid-based cytology has revolutionized non-invasive screening. The Pap smear, which evolved into HPV co-testing, identifies high-risk human papillomavirus strains in cervical samples, reducing incidence by 80% since its inception. Labs now amplify this with methylation assays, detecting aberrant DNA patterns in exfoliated cells for earlier precancerous lesion identification. For prostate cancer (299,010 projected U.S. cases), prostate-specific antigen (PSA) blood tests serve as gatekeepers, with thresholds adjusted by age and race, e.g., 2.5 ng/mL for African American men under 50, to minimize overdiagnosis. Elevated PSA triggers multiparametric MRI and targeted biopsies, where labs quantify Gleason scores via digital pathology.
Beyond solids, hematologic malignancies like leukemia rely on complete blood counts (CBC) with differential, flagging blasts or abnormal lymphocytes. Bone marrow aspirates, analyzed via fluorescence in situ hybridization (FISH), detect translocations such as BCR-ABL in chronic myeloid leukemia (CML), enabling tyrosine kinase inhibitor initiation. These foundational tests underscore labs’ diagnostic primacy: without them, the annual 1.9 million U.S. cancer diagnoses would plummet in accuracy, delaying interventions that save lives.

Biomarkers and Molecular Profiling: The Molecular Fingerprint of Cancer
What elevates lab diagnosis from descriptive to predictive is biomarkers, measurable indicators of tumor biology. In 2025, molecular profiling has become standard, with comprehensive genomic profiling (CGP) panels sequencing hundreds of genes from formalin-fixed paraffin-embedded (FFPE) tissue. For lung cancer (234,580 U.S. cases expected), EGFR, ALK, and ROS1 alterations guide osimertinib or crizotinib, respectively, improving progression-free survival by 18 months.
Protein-based biomarkers complement genomics. CA-125 for ovarian cancer (19,680 U.S. cases) rises in 80% of epithelial tumors, though specificity hovers at 70% due to benign elevations in endometriosis. Labs mitigate this via the Risk of Ovarian Cancer Algorithm (ROCA), integrating serial CA-125 kinetics with age. In pancreatic cancer, a notoriously lethal foe (66,440 U.S. cases), labs deploy CA 19-9 alongside NGS for KRAS G12D mutations, present in 90% of cases, to stratify resectability.
Circulating biomarkers herald a non-invasive era. Exosomes, tumor-derived vesicles in blood, carry miRNAs like miR-21, overexpressed in glioblastoma, detectable via qPCR assays with 85% sensitivity. For monitoring, tumor-informed MRD assays track patient-specific neoantigens, identifying relapse months before imaging. These profiles personalize care: a 2025 study showed CGP-directed therapies in 40% of advanced cases, versus 15% with standard histology alone.
Monitoring Treatment Response and Recurrence: Labs as Sentinels
Diagnosis is merely the opening salvo; monitoring ensures the war is won. Labs track response through serial biomarker surveillance and imaging correlates. In immunotherapy, a 2025 cornerstone for melanoma (100,640 U.S. cases), labs measure programmed death-ligand 1 (PD-L1) expression via IHC, with tumor proportion scores >50% predicting pembrolizumab efficacy. Post-treatment, rising lactate dehydrogenase (LDH) signals progression, prompting salvage regimens.
ctDNA monitoring shines in solid tumors. For colorectal cancer post-resection, Guardant360 assays detect ctDNA in 88% of relapsing patients up to 16 months pre-clinically. This “liquid vigilance” adjusts adjuvant chemotherapy, reducing overtreatment by 25%. In breast cancer, dynamic changes in circulating tumor cells (CTCs) via CellSearch, enumerating EpCAM-positive cells, correlate with visceral metastasis risk, guiding endocrine therapy intensification.
For hematologic cancers, quantitative PCR (qPCR) monitors BCR-ABL transcripts in CML, achieving major molecular response (<0.1% transcripts) in 70% on first-line imatinib. Flow cytometry tracks MRD in acute lymphoblastic leukemia (ALL), with levels <0.01% post-induction predicting cure. These metrics inform de-escalation: in Hodgkin lymphoma, PET-CT fused with lab FDG-PET biomarkers spares radiation in low-risk patients, cutting secondary malignancies by 15%.
Integration with electronic health records (EHRs) amplifies monitoring. Labs feed real-time dashboards, alerting clinicians to ctDNA spikes, which, in a 2025 meta-analysis, halved recurrence detection times versus CT scans alone.

Advanced Technologies Revolutionizing Lab-Based Oncology
The lab of 2025 is a symphony of innovation, where AI, microfluidics, and multi-omics converge. NGS, now processing whole genomes in hours for under $600, underpins precision oncology, identifying actionable variants in 60% of refractory cases. Paired with CRISPR-Cas9 for functional validation, labs edit tumor organoids to test drug sensitivities ex vivo.
Liquid biopsies dominate, with error-corrected ctDNA platforms like RaDaR achieving 99% specificity by distinguishing clonal hematopoiesis noise. For multi-cancer early detection (MCED), Grail’s Galleri test scans 50+ cancers via methylation patterns, detecting stage I-II tumors in 40% of positives.
AI augments pathologists: convolutional neural networks (CNNs) in digital slides flag micrometastases with 92% accuracy, reducing review times by 30%. Hybrid imaging, fusing PET with lab-derived radiomics, enhances prostate lesion detection. RNA tools scan tRNA modifications for pan-cancer signatures, while lab-on-a-chip devices miniaturize assays for point-of-care CTC isolation.
These tools democratize oncology: cloud-based NGS platforms enable low-resource labs to profile tumors, narrowing global disparities.
Case Studies: Labs in Action Against Cancer
Real-world vignettes illuminate labs’ impact. Consider a 59-year-old woman with metastatic breast cancer at Memorial Sloan Kettering Cancer Center (MSKCC). Initial biopsy revealed ER-positive, HER2-negative disease with PIK3CA mutation. Lab-directed everolimus plus exemestane yielded a partial response, monitored via serial CTDNA drops from 5% to 0.1% variant allele frequency (VAF), averting progression for 24 months.
In lung cancer, a Circulogene study profiled a non-small cell lung cancer (NSCLC) patient’s ARID1A and PD-L1 alterations from ctDNA, shifting from chemotherapy to pembrolizumab, achieving a complete response confirmed by PET negativity. Pre-treatment VAF was 12%; post-therapy, undetectable, with labs guiding six cycles before de-escalation.
For rare cancers, a UK case-control study analyzed pre-diagnostic bloods in 5,000 abdominal symptom patients, revealing elevated platelets and neutrophils in 70% of subsequent pancreatic diagnoses, prompting FIT and CA 19-9 protocols that expedited interventions.
In cancer of unknown primary (CUP), a 2025 Frontiers report detailed three cases: one ovarian-origin CUP identified via lab-based tissue-of-origin RNA assays (90% accuracy), enabling carboplatin-paclitaxel with 18-month survival. These stories underscore labs’ narrative power, turning data into destinies.

Challenges in Lab-Based Cancer Care
Innovation begets hurdles. Cost barriers loom: NGS panels average $5,000, unaffordable in low-income settings where 70% of global cancers occur. Screening inequities exacerbate late presentations; 2025 Quest Diagnostics data show oncologists noting 25% more advanced-stage diagnoses, blaming access gaps.
Accuracy falters in edge cases: ctDNA sensitivity dips to 50% in low-burden disease, risking false negatives that delay monitoring—rare biomarker validation stalls companion diagnostics, with FDA approvals lagging for <1% prevalence targets. Ethical quagmires abound, as incidental germline findings in somatic testing raise consent issues, while AI biases skew predictions.
Workflow pressures intensify: labs process 500 million U.S. oncology tests yearly, yet staffing shortages delay results by 20%. Overcoming these demands requires interoperable standards, subsidized tech, and diverse datasets.
Future Directions: Envisioning Tomorrow’s Lab Oncology
By 2030, labs will pioneer preventive oncology. AI-driven predictive models, ingesting multi-omics (genomics, proteomics, metabolomics), forecast risks from routine bloods, potentially averting 30% of cases. Companion diagnostics for ADCs like sacituzumab govitecan will expand, targeting TROP2 in triple-negative breast cancer.
Spatial transcriptomics will map tumor microenvironments, revealing immunosuppressive niches for novel immunotherapies. Wearables integrated with lab biosensors will enable continuous ctDNA tracking, flagging micro-metastases in real-time. Global trends forecast $300 billion in oncology R&D, prioritizing KRAS inhibitors and pan-cancer vaccines.
Equity initiatives, like WHO-backed NGS hubs in LMICs, will bridge divides. Automation and cybersecurity will safeguard data flows, ensuring labs evolve as resilient fortresses.
Conclusion
Laboratories are the linchpin of cancer’s defeat, forging diagnoses from whispers and sustaining remissions through vigilant oversight. From NGS unveiling mutations to ctDNA whispering of threats, labs empower precision that honors each patient’s unique biology. As 2025’s innovations, AI, liquid sentinels, and molecular cartography unfold, challenges like access and accuracy demand collective resolve. Investing in these epicenters isn’t an expenditure; it’s an oath to outpace cancer’s cunning, transforming statistics of despair into stories of survival. In the lab’s glow, hope isn’t abstract; it’s sequenced, quantified, and profoundly human.